The Toxic Twins
Carbon Monoxide & Hydrogen Cyanide

Carbon Monoxide & Hydrogen Cyanide
Introducing The Toxic Twins
Fire smoke has changed in the last 30 years. The extensive commercial and residential use of synthetic materials (plastics, nylons and polymers such as Styrofoam and polyurethane foam) has a significant impact on combustion and fire behavior, as well as the smoke produced during a structure fire. The majority of these materials are carbon based, bonded with various atoms like hydrogen, nitrogen, chlorine, and sulfur. Synthetic substances ignite and burn fast, causing rapidly developing fires and toxic smoke and making structural firefighting more dangerous than ever before. These materials ignite and burn 2-3 times hotter and faster than conventional materials and when heated, emit a gas or smoke that will also ignite 2-3 times faster and burn 2-3 times hotter.
There is little question that the today’s fire smoke is more deadly than ever before. Twenty years ago, firefighters came off of air as soon as the fire was out – or when they were outside of the structure. That is no longer possible if, as a firefighter, you want to prevent the life-ending or cummulative impact of these deadly gases.
Firefighters who breathe fire smoke and expose themselves to off-gassing during overhaul and salvage, are gambling with their hearts and brains. Every breath of fire smoke amounts to cummulative exposures, which over time, will debilitate the heart, brain and the central nervous sytem.
-
Carbon Monoxide adheres to oxygen binding sites in the blood with an affinity 220 times that of oxygen, thereby displacing oxygen from these sites
-
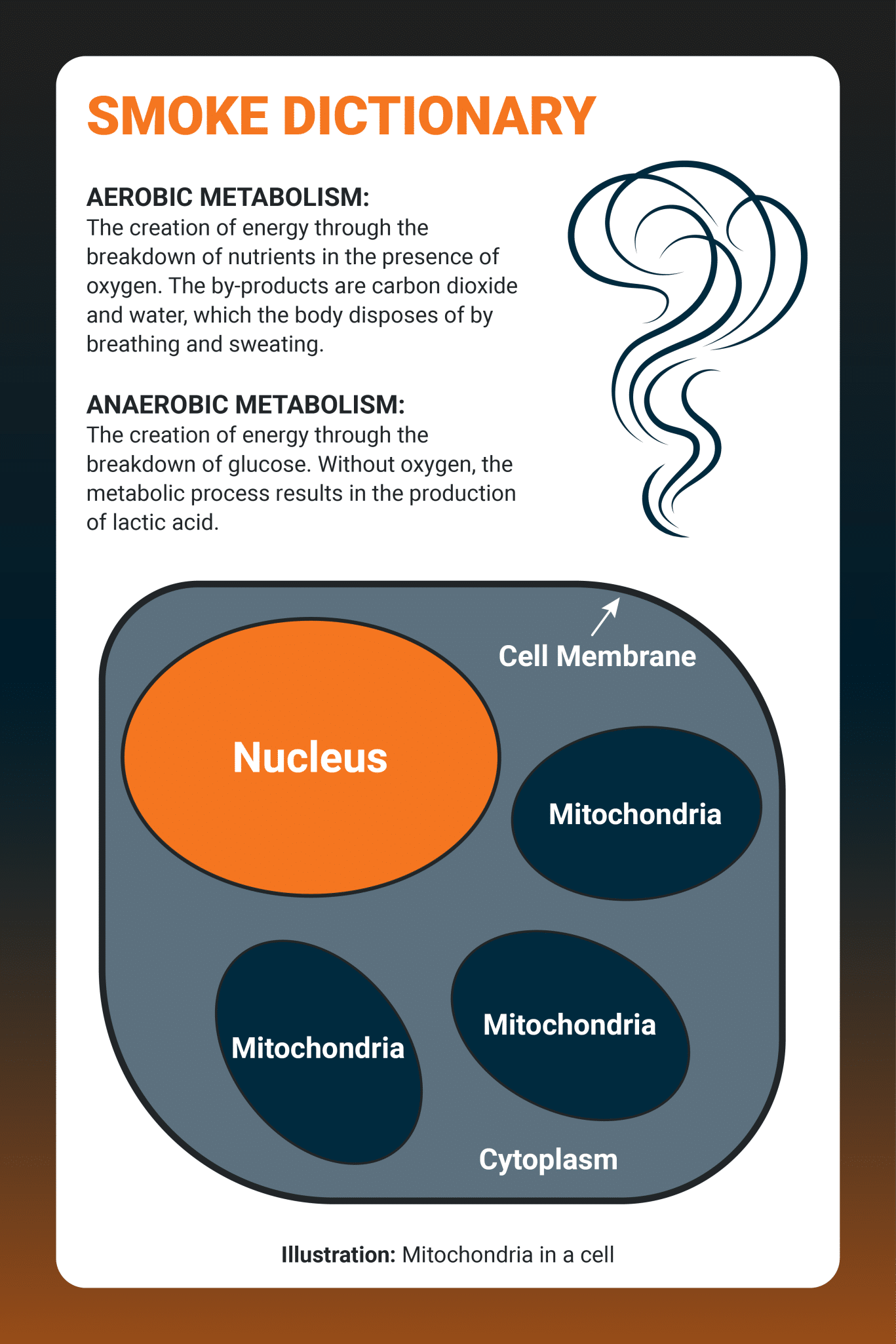
Hydrogen Cyanide disrupts the body’s ability to perform aerobic (oxygen utilizing) metabolism, even in the presence of normal oxygen levels.
This means that even if oxygen can be delivered to the cells, they are unable to use the oxygen and die anyway. Together the chemical asphyxiant effects of carbon monoxide and hydrogen cyanide are deadly. They are even more deadly in the presence of a low oxygen environment.
Resources
Carbon Monoxide
The reduction of the oxygen-carrying capacity of the blood leads to inadequate oxygenation of cells, tissues, and organs, i.e. “hypoxic stress”. The brain is especially susceptible to
hypoxia, but the subtle neurological effects of CO may prevent a victim from being aware of the danger. Eventually, brain function is depressed to the point where collapse and incapacitation occurs, followed by unconsciousness.
-
Carbon monoxide displaces oxygen in the red blood cells


Hydrogen Cyanide
Cyanide disrupts the body’s ability to perform aerobic (oxygen utilizing) metabolism, even in the presence of normal oxygen levels.
Once absorbed in the body, cyanide compounds ‘poison’ the cytochrome oxidase, barring oxygen from entering the mitochondria and effectively shutting down the process of aerobic metabolism.
Without oxygen, the cells switch to anaerobic metabolism, producing toxic by-products such as lactic acid, ultimately killing the cell. Therefore, cyanide toxicity is not about the amount of cyanide in smoke inhalation victims.
-
Hydrogen Cyanide prevents the cells from using oxygen
Preventing Exposure
add links to:
Why ROAM
Rule of Air Management
Importance of SCBA
More Air, More Time
Ric Jorge Breathing Techniques Video & Reilly Breathing Technique Videos
Link to Firefighter Rehab
Link to pre-hospital Treatment for Smoke Inhalation